News Story
Unveiling Nanoparticle Transport: Transforming Drug Delivery

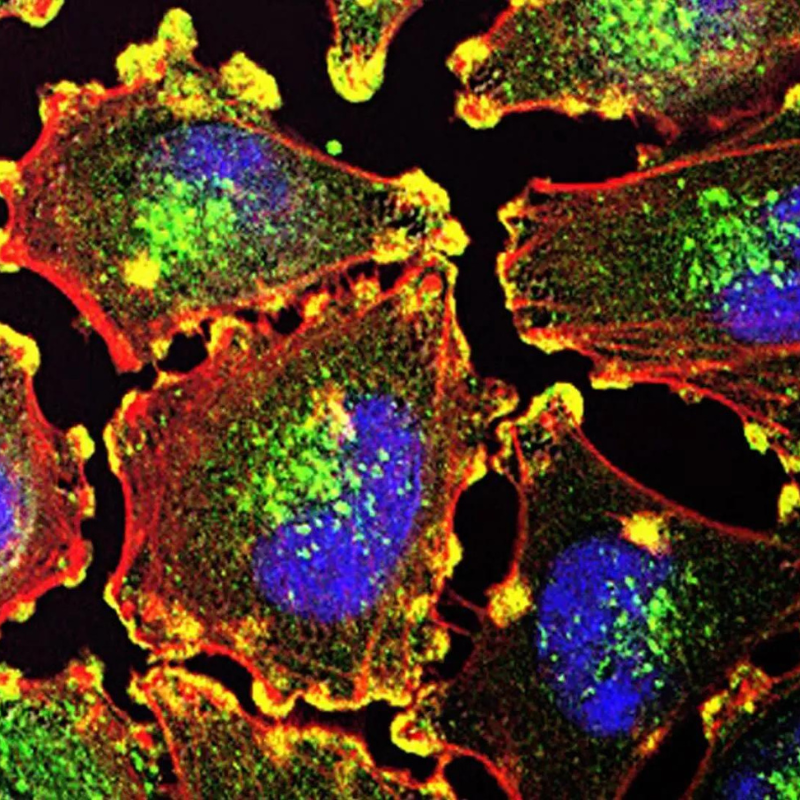
Nanoparticle-based drug delivery has emerged as a powerful strategy with diverse applications, from enhanced drug stability to targeted delivery to specific tissues. However, understanding how nanoparticles counter biological barriers, such as cell monolayers in blood and lymphatic vessels, remains a critical challenge. Researchers from the University of Maryland’s (UMD) Fischell Department of Bioengineering (BIOE) have made significant strides in understanding the intricacies of nanoparticle transport across lymphatic endothelial cells (LECs). This research, featured on the front cover of the peer-reviewed journal Molecular Pharmaceutics, showcases a pivotal advancement in the field of targeted drug delivery.
In their research, Computer Science and BIOE senior Jenny Yarmovsky, BIOE alumni Jacob McCright (Ph.D. ‘23), and Assistant Professor Katharina Maisel, took on the fundamental challenge of enhancing drug delivery efficiency by targeting nanoparticles to lymphatic vessels. Lymphatic endothelial cells serve as gatekeepers to the body's immune system, making them a prime target for modulating immune responses and delivering therapeutics effectively. By studying the transport kinetics of nanoparticles, the researchers aimed to uncover key insights into the mechanisms governing this process.
“The lymphatic vasculature is critically understudied and, even though there is an increased popularity to target lymphatic transport for drug delivery these days, we have incredibly limited understanding of how lymphatics transport nanoparticles and what the underlying mechanisms or kinetics are,” says Maisel, Principal Investigator of the Maisel Lab. “Understanding these will provide us better ways to intervene with therapeutics, particularly in disease settings where lymphatic transport and its mechanisms can be altered.”
In their research, the team elaborated on distinct transport mechanisms influenced by nanoparticle properties. Notably, inhibiting “exocytosis”—a cellular process involved in nanoparticle uptake and release—resulted in a significant reduction in transport efficiency. “Finding this through our research was notable, as it was confirmed by data in the literature already, which signifies how important those mechanisms are,” notes Yarmovsky, who also works as a Research Assistant in the Tao Lab.
Central to their findings is the discovery of the optimal size and surface chemistry of nanoparticles for efficient transport across lymphatic endothelial cells. Through in-depth experimentation and computational modeling, the team determined that densely PEG coated populated nanoparticles with a size of approximately 40 nanometers exhibit the highest transport efficiency.
These models offer valuable insights into the ideal surface chemistry, particle size, and other critical factors necessary for efficient drug delivery. By inputting specific details of a patient's condition or the requirements of a particular drug, these models could provide tailored recommendations for dosage and timing of drug delivery. This approach has the potential to optimize therapeutic outcomes while minimizing potential side effects and dose volume in drug distribution.
“Our findings shed new light into what drives nanoparticle transport into kinetics and suggests that—contrary to prior hypotheses, which suggested that nanoparticles are transported by purely passive paracellular pathways—nanoparticle transport is driven by transcellular mechanisms as well,” says Maisel. Throughout their research, they discussed the impact of fluid movement and disease on nanoparticle mechanism movement. Their aim is to utilize these findings to optimize strategies to enhance disease treatment strategies.
 Looking ahead, the researcher team envisions the development of comprehensive computational models that integrate various parameters to predict optimal nanoparticle properties for specific drug delivery needs. The ability to accurately predict the behavior of nanoparticles within the body could streamline the drug development process, expediting the translation of therapies from the laboratory to clinical use.
Looking ahead, the researcher team envisions the development of comprehensive computational models that integrate various parameters to predict optimal nanoparticle properties for specific drug delivery needs. The ability to accurately predict the behavior of nanoparticles within the body could streamline the drug development process, expediting the translation of therapies from the laboratory to clinical use.
Published May 3, 2024